
Student stress is well-documented within the field of simulation-based medical education. However, current research is unclear as to what level of stress is optimal for an enhanced educational experience. This ambiguity may partly be due to the use of one or a small number of stress metrics in study designs.
Our study will aim to evaluate the feasibility of measuring the human stress response during medical simulation, using a suite of techniques.
Audiovisual (video spectacles), biochemical (serum cortisol and plasma metanephrine/normetanephrine), physiological (blood pressure, galvanic skin response and heart rate) and psychological (State-Trait Anxiety Inventory and Big Five Inventory-2) markers of stress will be monitored during two randomized medical simulations of differing complexities.
Accounts of student stress are well-documented in the literature and are often associated with factors such as social evaluation (assessment in front of peers), the presence of senior staff and feelings of incompetence [1–3]. Simulation is a widely used training tool in medical institutions, where errors do not have the same clinical implications as in the real world [4]. However, simulation has the ability to evoke a plethora of emotional responses concerning the individuals taking part, all of which exert influences on learning and performance [5]. Research has shown that acute stress can either impair or enhance learning and performance, depending on the individual, the stressor and the individual’s appraisal of the stressor [6,7]. Opposing schools of thought argue over whether high-stress or low-stress environments are most suited to medical education applications [8]. Therefore, optimizing real-time stress in simulation environments may allow us to best equip medical students for the demands of their future careers.
To date, studies evaluating stress during medical simulation exercises have yielded varying results. A selection of studies appear to have success quantifying stress in terms of qualitative and/or quantitative measures [1,2]. For example, Mills et al . [1] report that salivary cortisol shows a significant increase in participants involved in medical simulations with three onlookers, on comparison with one. However, other authors report less satisfactory results [9,10]. For example, Stein [10] states that using heart rate variability (HRV) as an objective measure of stress does not determine any significant differences between simulation and control groups during emergency care exercises. Biochemical and physiological stress markers such as catecholamines, cortisol and HRV are often employed to build a picture of stress on an individual level. However, that picture is not always consistent. Most studies have focused on measuring one or a small number of metrics of the human stress response. The principle aim of this study is to evaluate the feasibility of measuring environmental stressors and individual stress responses during medical simulation, using audiovisual, biochemical, physiological and psychological markers.
This study will recruit final-year medical students at Queen’s University Belfast.
Due to the impact of a variety of medications on the autonomic nervous system, participants will not be considered for this study if they are currently taking medications which can affect autonomic nervous system activity and/or immune system responsivity, such as: tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin/noradrenaline reuptake inhibitors, α-adrenergic receptor blockers, β-adrenergic receptor blockers, calcium-channel blockers, monoamine-oxidase inhibitors, dopa-related drugs, stimulant/sympathomimetic drugs or oral estrogen, including estrogen-containing contraceptive pill.
Mr. Aaron Vage (AV) will act as study coordinator. Dr. Paul Hamilton (PKH) and Prof. Gerard Gormley (GJG) will measure blood pressure (BP), perform venepuncture and conduct briefings, simulations and debriefings. Dr. Gary McKeown (GM) will assist with statistical analysis. Dr. Andrew Spence (AS) will act as an auxiliary phlebotomist and simulation facilitator, when required.
A sample size calculation was completed, based on studies having previously evaluated heart rate (HR) during low- and high-stress simulations [1,9]. The number of participants dictated by the sample size calculation is 21 (n = 21). Variables used are as follows: µ 1 = 85 beats/min (mean of low stress), µ 2 = 95 beats/min (mean of high stress), σ = 13 beats/min (common standard deviation).
Participants will be recruited via poster advertisement and targeted email. If the proposed recruitment strategy fails to generate sufficient interest in the project, the recruitment campaign will be extended as follows:
A member of the research team will seek permission to make an announcement at the start of a lecture/class that final-year medical students are attending. Project supervisors (who are staff members) will not be involved in making such announcements to remove any perception of coercion. A member of the research team will approach the QUB medical student society and seek permission to engage members about the study. Project supervisors (who are staff members) will not be involved in making such contact. Snowball sampling: this method of recruitment may offer a sizeable benefit in terms of study participants advertising verbally to other potential participants. Social media: the Centre for Medical Education has an active Twitter account engaging a sizeable student audience. Permission may be sought to share the study recruitment poster using this account.
Participants will attend the KN Cheung SK Chin InterSim Centre at Queen’s University Belfast. On arrival, participants will be welcomed and asked to complete the State-Trait Anxiety Inventory (STAI) and the Big Five Inventory-2 (BFI-2). After instruction, participants will retreat into a private room and fit themselves with a chest strap device (Equivital EQ02+ LifeMonitor, UK) that will monitor HR and galvanic skin response (GSR). After device fitting, BP will be taken by an automated sphygmomanometer (A & D Medical, Japan), with a cuff on the upper arm, and blood samples (for serum cortisol and plasma metanephrines) will be collected, using a protocol provided by the hospital laboratory who are performing sample analysis. Finally, participants will be fitted with point-of-view (POV) spectacles (Oho Video Glasses, China) and briefed by an experienced medical professional, before engaging in the first simulation (Figure 1).

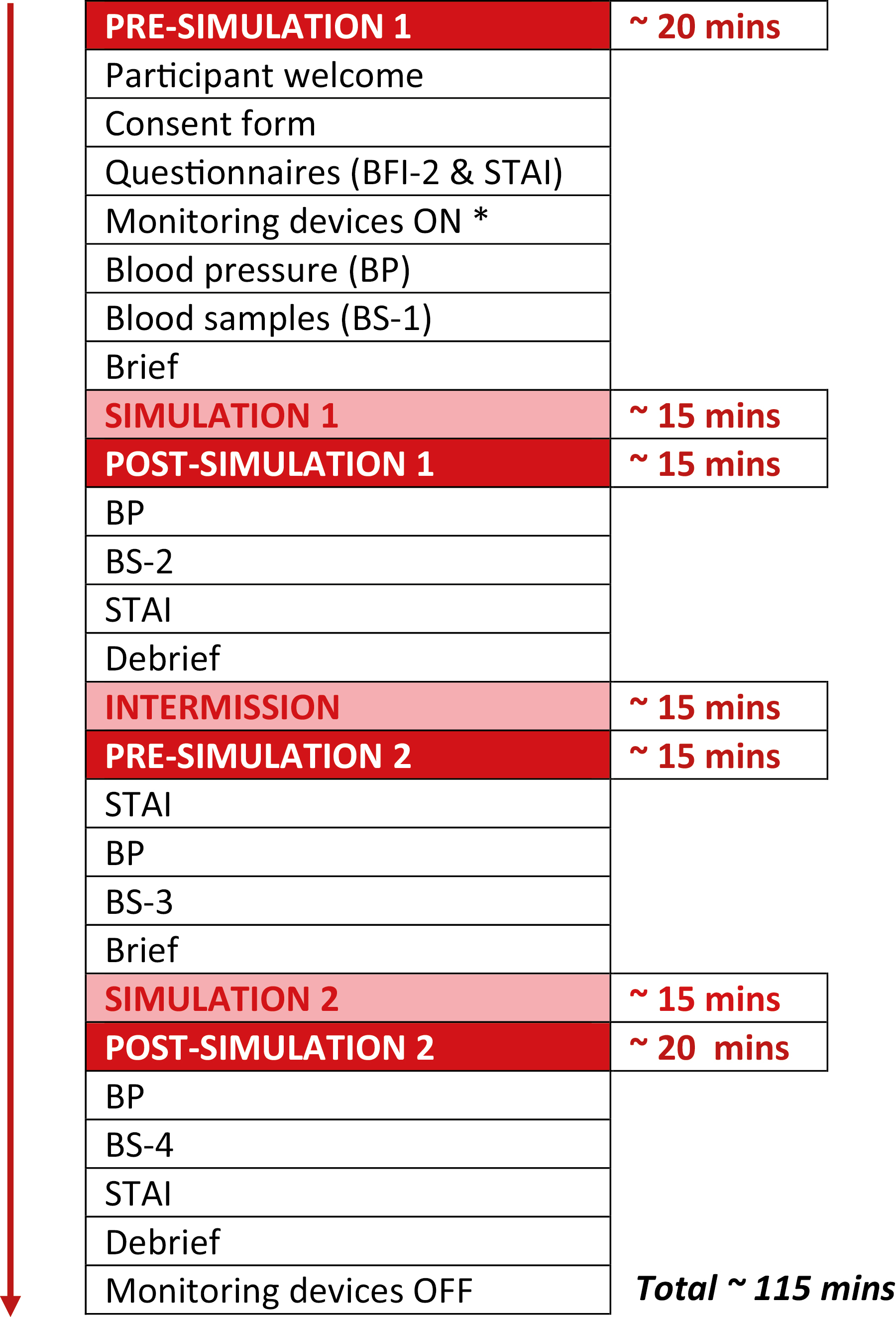
Schematic of study. * EQ02+ LifeMonitor (Equivital, UK), Video Glasses (Oho, China).
Participants will complete two medical simulations (one low-complexity, one high-complexity) in a random order. A random number generator will be used to determine which simulation participants undertake first. The low-complexity scenario will involve the participants treating a patient suffering from mild asthma exacerbation. The high-complexity scenario will involve the participants treating a patient suffering from life-threatening asthma. Scenarios have been scripted and will follow the same course for each participant.
Immediately after simulation 1, participants will, again, complete the STAI, and BP will be taken. Blood samples (as before) will be collected after BP measurement. Participants will then be debriefed by the researcher who completed the simulation. Participants will then be allocated a private room and asked to remain seated for a 15-minute intermission period. On completion of the intermission period, participants will complete the STAI, and BP will be taken. Blood samples (as before) will be collected after BP measurement. Participants will then be briefed before engaging in the second simulation. Immediately after simulation 2, participants will complete the STAI, and BP will be taken. Blood samples (as before) will be collected after BP measurement. Participants will then be debriefed. Post-debriefing, participants will be asked to retreat to a private room and remove both the chest strap device and POV spectacles. This will conclude participant involvement.
Participants will complete both the STAI and BFI-2 during the study. The STAI has been selected as a measure of subjective stress due to its successful use in investigating the impact of stressful environments on self-reported stress [4,10–12]. As the STAI focuses on the present, it will allow analysis of perceived participant anxieties pre- and post-simulation. The BFI-2 will give numerical outputs regarding the participant’s levels of agreeableness, conscientiousness, extraversion, neuroticism and openness [13,14] – having been selected as a personality metric due to its wide use in studies evaluating the relationship between personality traits and human stress responses [15–19]. This will permit evaluation of participants’ personality traits, with the aim of comparing them to data from the STAI, and biological markers of stress.
The Equivital EQ02+ LifeMonitor will monitor participant HR and GSR for the duration of the study. During the human response to stress, both HR and GSR have been shown to significantly increase from baseline values [1,20]. The stress-mediated increase in HR is due to the positive chronotropic effects of adrenaline and noradrenaline on the heart, allowing more nutrient-rich blood to reach targeted tissues in anticipation of work. GSR refers to variations in the electrical activity of the skin, which typically occur during physiological stress due to increased sweat production. This activity may be measured by applying an electrical current to a region of skin, between two electrodes, and analysing electrical conductance. Acetylcholine is the predominant mediator of eccrine sweating during periods of acute stress, driving the rise in GSR [21]. Therefore, an increase in conductance would indicate elevated sympathetic nervous system activity in response to a stressor.
BP will be measured at four time points throughout the study. During the human stress response BP increases, due to the vasoconstrictive, as well as the positive chronotropic and inotropic effects of adrenaline and noradrenaline on the arteries and heart, permitting the delivery of blood at higher velocities to working musculature.
GSR, HR and BP have been selected as physiological stress markers due to their non-invasive nature and successful use in studies examining the effects of stress on the physiological manifestations of the stress response [1,22–24].
Participants will be required to give four blood samples, collected at specified times throughout the study. Samples will be analysed for the catecholamine metabolites, metanephrine and normetanephrine, as well as cortisol. Plasma metanephrine and normetanephrine have been selected as biochemical stress markers of the sympathetic–adrenal–medullary (SAM) axis, due to the unstable nature of their parent molecules [25,26]. Serum cortisol has been selected as a biochemical stress marker of the hypothalamic–pituitary–adrenal (HPA) axis, due to its wide use in studies pertaining to psychological stress [27,28]. As products of the human stress response, the aforementioned molecules are often considered to be the gold standard metrics concerning mammalian biochemical stress analysis [29].
Participants will be fitted with POV spectacles, which will record the simulation from their visual perspective. The spectacles will be fitted prior to simulation 1 and removed after debriefing for simulation 2. Audio from the footage will be analysed by emotive speech software (openSMILE 3.0), which will evaluate the participant’s language, speech tempo and word repetition. This form of analysis may reveal stress-related word patterns and language architecture that can be compared with the biochemical, physiological and self-reported data – revealing possible themes, words or phrases that are used as a participant perceives their immediate environment as increasingly stressful.
The primary outcome of the study is to report on the feasibility of collecting the data as described. Field notes will be collated at the end of the study in this regard. However, Wilcoxon signed-rank tests will be employed to analyse any quantitative data collected during the study. P-values less than 0.05 will be considered to indicate statistical significance; all tests will be two-tailed. Analyses will be performed using SPSS/PC (Version 27, SPSS Inc., Chicago, IL, USA).
Blood sampling will be performed by medically qualified personnel with experience in this skill. Any student suffering ill effects (e.g. fainting) from this procedure will be tended to immediately by this doctor. A decision on whether or not to continue with the study will be made in agreement with the student. Medical simulation events can evoke a range of emotions for students. In the unlikely event that a student experiences psychological distress, a ‘distress protocol’ will be invoked. Further participation in the study will be stopped and the student will enter a controlled debriefing environment with an experienced simulation facilitator. Should a participant have a BP at trial entry that is in the range suggesting hypertension (i.e. greater than 140 mmHg systolic and/or greater than 90 mmHg diastolic), and this pressure is maintained throughout the study, a medical professional (PKH) will explain the findings to the student in a confidential manner and inform the student’s General Practitioner by letter.
The quantities of several chemical mediators are being measured in blood in this study, and there is a small possibility that a participating student might incidentally be found to have an abnormally high or low concentration of one or more of these markers. Such findings will be noted after the study day when results are returned from the laboratory. As both cortisol and metanephrines are normally measured under controlled circumstances with efforts made to minimize stress, only baseline results will be compared to laboratory reference ranges. It is expected that the psychological stress induced by the simulation will increase these mediators, rendering the reference range meaningless. Should a subject’s baseline blood results fall outside the reference range, a medical professional (PKH) will explain the findings to the student in a confidential manner and arrange any necessary follow-up in the Belfast Health and Social Care Trust, keeping the student’s General Practitioner apprised of the situation.
Student performance will not be formally assessed or recorded and will therefore have no bearing on their academic record. However, in the unlikely event that a participant’s performance raises concerns about their competency, a member of the research team involved in undergraduate medical education (PKH or GJG) will speak to the student in question and arrange any remedial activity as deemed appropriate.
The following results and/or outcomes are anticipated:
Participants will report higher levels of anxiety/stress post-simulation, on comparison with pre-simulation, via the STAI – self-reported anxiety/stress levels will be highest after the high-complexity simulation. Higher self-reported anxiety/stress scores will predict higher stress responses (for all metrics). Higher neuroticism scores will predict lower serum cortisol responses, whilst higher extraversion scores will predict higher serum cortisol responses. Mean HR and GSR will increase during simulation – mean HR and GSR will be highest during the high-complexity simulation. Additionally, peak HR and GSR will be highest during the high-complexity simulation. BP will rise after a simulation and will be highest after the high-complexity simulation. Plasma metanephrines will be higher post-simulation than pre-simulation – plasma metanephrines will be highest after the high-complexity simulation. Serum cortisol will be higher post-simulation than pre-simulation – serum cortisol will be highest after the high-complexity simulation.
AV – conceptualization and design of study, major contributor to manuscript. GJG – design of study, contributor to manuscript. GM – contributor to manuscript. PKH – design of study, contributor to manuscript.
Partly funded by the Northern Ireland Department for the Economy postgraduate studentship program.
None declared.
Full ethics committee approval has been obtained. Full, written informed consent will be obtained from all subjects.
None declared.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.