
Pleuroscopy is a safe and sensitive alternative to video-assisted thoracic surgery for the diagnosis and management of malignant pleural effusion. Pleuroscopy requires fewer resources and can be offered to patients with reduced surgical fitness. A healthcare re-design project was required to establish pleuroscopy in our hospital system. These projects improve the quality and accessibility of care for patients and often result in multiple changes occurring simultaneously within a complex system. The Systems Engineering Initiative for Patient Safety model highlights the system elements that may be impacted when considering system redesign such as the environment, people/roles, tools/technology, tasks and organization. The resulting impact to our processes, patient/staff safety and desired outcomes is not always predictable when changing one or several elements.
Simulation is a key method to integrate into redesign projects to ensure the preparedness of staff, systems and processes involved, although it isn’t always utilized. This redesign involved relocating pleuroscopy procedures from the operating room (OR) suites to an outpatient bronchoscopy suite. Short skills-based simulation sessions (i.e. sub-sections of the workflow) were included for learning specific skills, followed by team simulation events as a final implementation step to ensure readiness. Based on this approach, restructuring of process, team roles, the environment, equipment and more was evaluated using simulation to test each system element undergoing change.
Simulation provided an essential means to evaluate staffing and roles (i.e. expanded scope of practice for respiratory therapists and nurses); the development of cognitive/visual aids and checklists; policy changes; initial staffing modifications, standardization; environmental changes; process changes and more. During the first year since implementation, 25 pleuroscopy procedures have been successfully completed without any safety events reported.
Systems testing and education using simulation was required to ensure an effective implementation and reinforce the many redesigned elements. Simulation was able to proactively test how this procedure could be achieved safely in the new environment. This article serves to demonstrate the utility of simulation for systems testing and staff training for a large system redesign project moving a diagnostic procedure from the OR to an outpatient bronchoscopy suite.
Healthcare re-design involves making multiple changes to practise that improve quality, reduce cost and improve the care provided to patients. Healthcare simulation is increasingly used to support system change by enabling proactive testing in situ (i.e. in the real clinical environment) without risk to patients (1,2). Systems-focused or translational simulation moves beyond simulation to primarily train and educate staff and focuses on testing the systems and processes of care to improve quality and safety (3–12).
For patients with suspected metastatic or advanced thoracic cancer and a new pleural effusion, a biopsy of the pleura may be necessary to establish the diagnosis. Biopsy performed under direct visualization using a video-assisted thoracoscopic surgery (VATS) is considered the gold standard although access is impacted by a patient’s surgical fitness, operating room (OR) time and surgical staff availability (13–15). Pleuroscopy is similar to VATS pleural biopsy but can be safely performed in select patients with reduced surgical fitness in an outpatient procedure environment without the need for OR staff and anaesthesiology (13–15).
Pleuroscopy was recently introduced in our hospital to help reduce pressure on surgical services, reduce cost, and improve access to timely and safe pleural biopsy. We leveraged existing staff in their previous roles and expanded the scope of practice for registered respiratory therapists (RRTs), teaching them new skills such as scrubbing and circulating that were traditionally only performed by nurses in the OR. The Systems Engineering Initiative for Patient Safety (SEIPS2.0) framework is a Human Factors model that describes the system elements to consider when improving the work of healthcare professionals and patient care (16). Elements such as the physical environment, roles/tasks/people, tools, technology and organization are all integrated parts of the healthcare system and changes to any one of these may impact process and patient outcomes.
The goal of this article is to describe how we used simulation as a key implementation approach for the successful transition of VATS pleural biopsy out of the OR and into an outpatient bronchoscopy suite without increasing human resources and while considering the many system elements to ensure a safe and sustainable transition.
We employed two strategies to estimate the need for pleuroscopy. We used published population-based data from comparable health systems to estimate potential needs, and we reviewed historical data from our provincial health database.
There are no large epidemiologic studies of the incidence of malignant pleural effusion (MPE) in Canada. The 2010 British Thoracic Society guidelines estimate the annual incidence of MPE in the UK to be 80 cases per 100,000 population (17). This number is extrapolated from several US studies representing a variety of population demographics over time. The catchment population for our hospital for specialized services was 1.71 million in 2020 with a median age of 38 years and 13.2% of the population over the age of 65 years. If the average sensitivity of thoracentesis for the diagnosis of MPE across all cancer types is 65%, we expect approximately 1350 new MPEs per year, with about 475 remaining cytologically unconfirmed after one thoracentesis, if all effusions were sampled. However, it is difficult to estimate how many patients with MPE would benefit from pleuroscopy because (1) not all patients with MPE require pleural sampling/drainage or require a confirmatory biopsy if pleural cytology is negative, (2) data from high-volume centres suggest that up to 50% of patients who undergo pleuroscopy actually have a benign effusion, even in a population with a high prevalence of malignancy, and (3) pleuroscopy is also indicated for select patients with suspected benign conditions, such as tuberculosis, which are not captured if only the incidence of MPE is considered (18).
Historically, there was an average of 12 VATS pleural biopsies per year performed in our hospital between 2016 and 2019. These years were chosen as they represent typical years prior to the availability of pleuroscopy and the impact of the COVID-19 pandemic. For the fiscal year 2017–2018, 10 VATS pleural biopsies were performed, 18 patients were referred but VATS was not performed, and 65 non-surgical closed pleural biopsies were performed, many of which were non-diagnostic. Patients with non-diagnostic biopsies were not always referred for VATS pleural biopsy for a variety of reasons, including lack of surgical fitness.
Based on this data, we estimated around 15–40 patients per year would be appropriate candidates for pleuroscopy in our catchment area.
Wait times were estimated from a provincial database, the Analytics Operating Room Repository. Data in this repository use Coding Access Targets for Surgery, a standardized coding system to measure wait lists for surgery. The median wait time for VATS pleural biopsy was between 9.5 and 22 days, depending on the year between 2016 and 2019.
Cost estimates for VATS pleural biopsy and pleuroscopy were estimated by the Provincial Health Services Financial Analytics Department and the Activity Costing Finance Department. Since pleuroscopy was not previously performed in our hospital, pleuroscopy cost was estimated by (1) using as a proxy day surgical procedure that is similar in resource requirements (bronchoscopy) and (2) building up the cost using a resource list. We estimated that pleuroscopy would be about $6000 cheaper per case, on average. The estimate was highly sensitive to the length of stay.
Note that cost estimates do not include physician professional fees as physician remuneration schemes vary in our health region. Since pleuroscopy in the ambulatory setting employs non-surgical specialist physicians and does not require an anaesthetist, health systems savings are likely underestimated.
The net new one-time start-up costs for equipment was approximately $50,000 based on vendor-provided invoices. Pleuroscopy procedures were scheduled during available endoscopy time and utilized existing staff and resources. Only the additional capital costs for the pleuroscopy procedure were considered when determining new and recurring costs, since the cost of operating our existing procedure area are fixed.
Scope of practice is defined as the accepted roles and responsibilities within a given profession based on regulatory bodies and organizational policies and guidelines (19). It was anticipated early that the integration of simulation methods would be key to train and educate staff on this expanded scope of practice (i.e. new tasks, new procedure) as well as testing the environmental layout and processes involved. Given the current ‘pre-project’ roles of the RRT and RN staff in the bronchoscopy suite, it was anticipated that the scope of practice would be expanded from current practice whereby an RRT would perform traditional RN roles of ‘scrubbing and circulating’ that would require a new and focused educational curriculum including the use of simulation for pleuroscopy procedures. The role of the RN would also expand with a greater focus on the head of bed monitoring during procedural sedation.
Early in the planning phase, it was identified that a lead RRT supervisor would be a necessary resource to utilize for educators, staff, physicians and leadership. The role was instrumental in planning, preparing, implementing and ongoing success of the project. The role was utilized as a participant in the 2-day training, and provided ongoing simulations for staff after the official training to ensure staff comfort and competence. This role continued to support staff post-implementation with regular training sessions for those who did not frequently have exposure to the procedure’s workflow. It was determined that the RRT supervisor should be present in the room for the first several procedures as an extra resource and provide staff with greater confidence and support to ensure sterile fields were maintained. The role was an instrumental part in scheduling the procedures in the beginning as this ensured the appropriate staff were available for the day of the planned procedures.
As part of the operational plan to relocate pleuroscopy procedures from the OR to bronchoscopy procedure rooms, the Clinical Nursing Educator was contacted to devise a plan to support educational programming for the RRTs. A project working group was created that included a manager, unit manager, RRT supervisor, provincial OR educator, and clinic educator. Ad hoc members included a simulation lead and physician lead for pleuroscopies in the outpatient setting.
The project followed the successful completion of the “A Project Ethics Community Consensus Initiative – ARECCI” screening tool (https://albertainnovates.ca/arecci-decision-support-tools/). This decision support tool identified the primary purpose of the simulation project as quality improvement and that the project involves minimal risks; therefore, a review by the Research Ethics Board was not required.
Critical consideration to the success of the project was the assurance that the outpatient bronchoscopy suite environment would meet infection, prevention and control (IP&C) requirements including airflow, correct placement of equipment (e.g. hand hygiene stations, surgical protective equipment) and identifying any areas of risk within the procedure room. The project team leadership (i.e. leading pleuroscopy physician) consulted with the IP&C team to review the environment and evidence. It was determined that based on the nature of the procedure, the airflow could remain the same (i.e. was set to negative airflow pressure instead of positive airflow such as in the OR environment) as the risks for surgical site infection were minimal. The follow-up from this equipment and environmental review led to the instalment of an antiseptic hand preparation solution in the anteroom, visual aids to help with hand scrubbing including a mirror to assist with donning, and additional supplies needed to comply with hand scrubbing techniques as per the Operating Room Nurses Association of Canada (ORNAC) (20). These hand hygiene locations, visual aids and a mirror were all tested as part of the simulations with staff.
To ensure a clear and accurate transition to the new environment, a direct collaboration and a 1-day observation between the OR staff and outpatient staff was facilitated. This work included the integration of a new rigid scope and its use in the procedure room. Other equipment required (i.e. surgical back table and positioning devices) was reviewed, including the medical device reprocessing requirements and process including how to build new instrument trays and the logistics of equipment location and sending. The observation day in the OR informed the type of drugs (i.e. talc, lidocaine with epinephrine) that needed to be available to perform the procedure that would not be typically found in a procedure room. For example, local anaesthetic medication for the surgical incision was not present in the procedure room; therefore, it was added to the ward stock. The steps to add a medication included a formalized request and procedure change to ensure that errors were minimized given the similarities of the drugs used daily.
Given all the changes in processes and procedures, a site-specific policy was drafted and created to support the changes. The policy outlined the newly expanded scope of practice of the RRT and the nurses related to the procedure. It included the equipment list and a description of the procedure steps. Medical Device Reprocessing Department processes are highlighted in the document to standardize the instrument handling process. The policy also included emergency processes that need to be implemented for a pleuroscopy procedure in the new environment. All of these learnings were applied in the training and simulations.
Consultation was provided upon request by additional clinical practice consultant services from the peri-operative environment to support OR-focused curricula. Together with the project working group, a condensed 2-day workshop was developed focusing on the new procedure. The objectives of the workshop focused on the new skills of scrubbing, circulating, setting up equipment, best practices to maintain sterility and procedure flow, and testing the workflow from beginning to end.
The first day was focused on foundational skills including equipment review, donning and doffing Personal Protective Equipment (PPE) and room set-up. The day began with didactic learning to understand the theory and foundations for sterile technique related to pleuroscopy procedure. Skills were broken down by workflow steps and practised via short skill-focused simulations until learners met the criteria of competency based on best practice standards from ORNAC guidelines and observation from the OR subject matter expert (20). The ORNAC guidelines were used as a reference point to determine the appropriate sterile technique (20). Competency to perform a task was based on how well learners adhered to the standards with little to no prompting from the OR educator. Observation by the OR educator was one approach used to determine whether the learner could move on to the next task based on the criteria set out by the standards, and replicated routine training practices in the OR. The skills included gloving and gowning, doffing and donning, opening sterile packaging and maintaining integrity, principles of sterile technique, back table set-up, sterile medication administration, sharps handling and draping. In the afternoon, equipment and instrumentation review was completed along with trialling room set-up and practising patient flow. The second day was focused on in situ simulation of the new procedure with all interprofessional team members. The project team had collaboratively developed two simulation scenarios for the team. The simulations included a routine ‘day in the life’ pleuroscopy procedure workflow with a patient coming into the outpatient suite, undergoing routine sedation and pleuroscopy until completion and preparation for transport. The second scenario was focused on an emergent patient deterioration mid-way through the case where the patient suffered increased blood loss requiring the surgeon, and the progression to a cardiac arrest situation. In both scenarios, one of the project team members acted as a patient and soon after arrival to the room underwent a simulated conscious sedation to begin the procedure. Each simulation was followed by a debriefing used to collect feedback on the system elements including room set-up, learner questions, tasks and process (4). User feedback was captured, summarized and used for ongoing improvement measures. Self-awareness of any breaches to sterile technique and self-confidence for the learner and team was another indicator for system evaluation. Together, the learners and content experts identified gaps and areas for improvement that were captured and summarized for follow-up.
The overall project timelines are highlighted in Figure 1. As a consequence of the COVID-19 pandemic, the span of planning and execution took much longer than anticipated due to shutdowns and limited social gatherings.

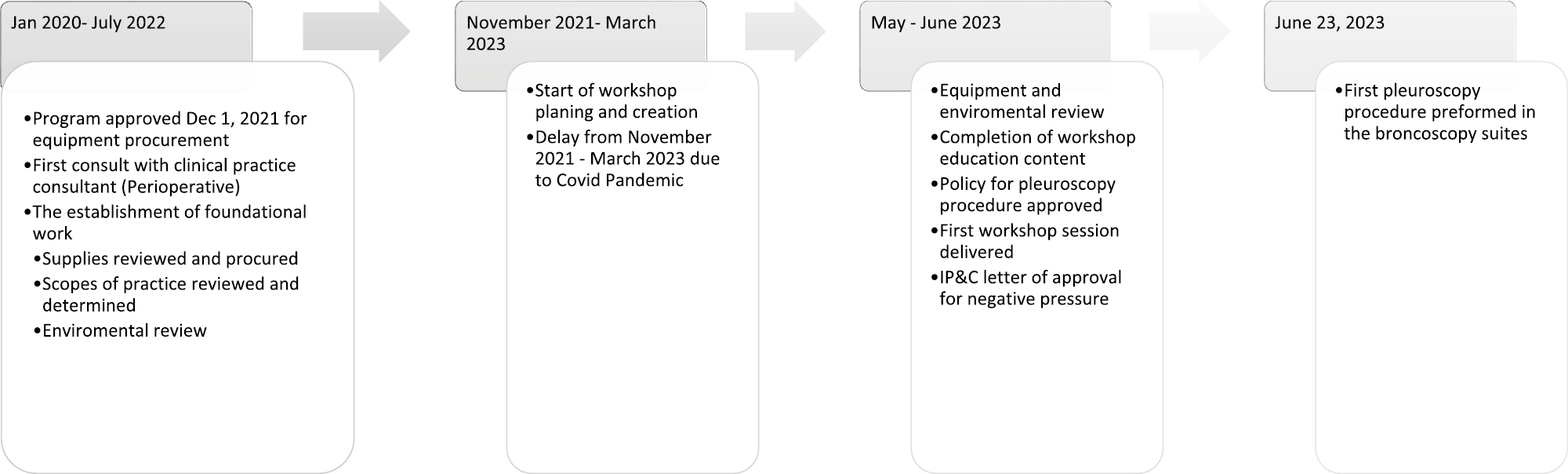
Project Timeline
Table 1 outlines the systems-based findings and outcome measures that resulted from the education, training and systems simulation testing programme prior to launch.

| System category | Category description | Findings from simulation | Improvement or mitigation strategies resulting from simulations |
|---|---|---|---|
| People/roles/tasks | Staff, patients – who is impacted, what is required of them in their workflow. Consider complexity, sequence, ambiguity as examples | Training in new tasks and roles • RRTs expressed the need to practice scrubbing and circulating roles as an expanded scope. For example, sterile consciousness awareness, scrubbing and circulating role practice • Supervisor/educator coaching of staff on body positioning with PPE donned in relation to room • Entire new room set-up – RN required solely to monitor patient condition behind sterile drape (formerly MD role) • RN performing some traditional RRT tasks: suction, oxygen therapy and charting on behalf of RT for samples etc. • Physician role – coaching team on the new table set-up and procedural sequence, emergency management tasks • Consolidation of skills, build confidence and competence (e.g. donning of PPE, handing of equipment) |
• Additional practice sessions and stations made available for staff to practice on an ongoing basis. • Leading resource role of RRT supervisor to enable staff practice and feedback sessions. RRT supervisor to be present for first few procedures as resource and to aid in maintaining sterile field. • Visual aids created to support back table set-up • Policy changes on the role of the RN to support the patient during conscious sedation – an RN-specific flow document created to guide the RN role and responsibilities • Established room set up instructions for future procedures based on the simulation experience |
| Process | Consider what processes have been changed, removed, added, impacted as examples | • Challenges with traffic control during sterile procedures • Scheduling conflicts and time management noted during set-up and simulated practice • Medical device management for calling sterile trays from MDRD, proper storage for sterile integrity and timely access |
• Signage created and displayed on the outside door of the procedure room to notify sterile procedure in progress and access points for entry during this time • Pleuroscopy procedures booked on a dedicated day of the week and time. Additional time is held to allow for set-up time and appropriate case length. • RRT supervisor responsible for equipment handling and maintenance |
| Environmental | How does the environment impact them in their role. Consider lighting, noise, distraction, physical layout, available space | • Room set-up – complete with sterile procedure tables – tested and improved through simulation to reduce contamination risk • Extra equipment found congested the room limiting movement and increasing risk of sterile back table contamination • Emergency equipment carts for RRT and RN were difficult to access or not as easily accessible for time-sensitive situations • Risk discovered in simulation of staff head bumps/head injury from overhead monitor placement • Achieving optimal hair coverage and face mask placement required assistance of a mirror |
• Standardized room set-up was established based on the outcomes of the simulation • Extra equipment removed from room to help with ease of flow • Cautery placed in anti-room for quicker access in an emergency • Emergency equipment carts for RRT and RN were rearranged in room to improve access • Back table set-up depicted through a series of labelled photographs on the wall for easy reference. Photographs made to show how to assemble suction controls, biopsy forceps, case tray. Names of equipment labelled to aid in identification. • Checklists developed for procedure equipment preparation • Storage bins for equipment created for organization and access to improve efficiency • Addition of mirror in prep room for hand scrubbing station |
| Tools/technology | Consider equipment, resources, IT, objects they use or that assist them in doing their work, level of automation, usability, accessibility, functionality, etc. | Cognitive aid-cart set-up (pics) • Unable to quickly access emergency numbers for thoracic surgeon on call • Order of set-up and standardized set-up difficult to recall. • Documentation questions and specifics in simulation. • List of supplies difficult to build for preparatory equipment • Recalling steps for donning/doffing and instrument set-up is difficult to achieve for novice learners • Patient volunteers voiced discomfort during positioning from positioning equipment |
• Emergency phone numbers for thoracic surgeon on call added to phone as a cognitive aid • Back table set-up depicted through a series of labelled photographs on the wall for easy reference. Photographs made to show how to assemble suction controls, biopsy forceps, case tray. Names of equipment labelled to aid in identification. • Reference sheet for electronic health record system for nurses added to EHR station • Prep equipment now includes a sterile bucket, and a non-sterile bucket. Each includes a content list. • Ordering lists created for special equipment that is not routinely stocked in the bronchoscopy suite. • Established practice stations for ongoing practice following simulation feedback on: PPR donning/doffing and procedural scope set-up • Positioning equipment modified through iterative testing to improve patient comfort and the prevention of pressure points |
| Organization | Consider staffing, workloads, schedules, assignments, policy and procedures, education and training, work culture, resource availability, management and incentive programmes as examples. | • Conflicts found in hospital policy related to resources management and human resources • Tasks for completion during simulation were found overwhelming for staffing model • Concerns with retention of skills and sustainability for RRTs • The length of pleuroscopy cases was uncertain and/or unknown based on the learning curve of the staff |
• Policy edited to correct conflicting practices with pleuroscopy procedure • Staffing supported need for three staff working for initial procedures. • Adjusted booking process to ensure procedure time is sufficient and appropriate staff available for procedure. • Changes made to the policy included the following: procedure steps, equipment needs, emergency management and resources for sterility management • RRT supervisor provides ongoing teaching. This includes intraoperative support for staff will less experience. • Staff rotate into cases to maintain skills by being reassigned from other areas. • Confirmed procedure length and established plan for bookings |
Since implementation, and at the time of this article’s writing, 25 patients have undergone pleuroscopy in our outpatient setting between June 2023 and June 2024. There have been no adverse events or safety concerns reported during the first year. It was decided at implementation to obtain staff feedback following each procedure including a review of what went well and anything that could be improved. The RRT supervisor remains in attendance at cases and tracks any changes or improvement suggestions. Ongoing simulation programming ensures staff comfort and competency and ensures regular exposure through simulation practice.
Our project highlights an effective and safely launched system redesign project, moving a diagnostic pleuroscopy procedure from an OR location to an outpatient bronchoscopy suite. Although internal data had limited estimations to measure the exact impact on wait times and organizational cost, for example, this redesign project was supported by the organization as a reasonable means to benefit patient access to care, reduce loads on surgical wait lists and as a less expensive means of performing this diagnostic procedure outside of an OR. In addition, it was advantageous to harness the expanded scope of practice of health professionals that were available within the system to achieve this redesign.
The SEIPS 2.0 human factors framework highlights that when changing multiple system elements in a complex system, that the resulting impacts to other elements may not be fully understood until implemented including any latent conditions that may result in unintended patient harm. The use of simulation in tandem with the other educational and project planning that occurred, was key to understanding how this implementation would unfold in the outpatient setting including all of the changed system elements such as the new environmental layout and location, expanded skill sets and roles, and the need for new tools (i.e. cognitive/visual aids and checklists), equipment, organization (i.e. policy, staffing) and processes. Simulation enabled both the training of staff as well as testing of the processes and systems that surrounded the teams. In addition, simulation was front and centre for ensuring ongoing skill development.
Often eliciting this ‘work as done’ is achieved using experiential methods such as simulation versus other methods that don’t easily allow for teams to work in the in situ clinical environment while ensuring no risk to actual patients during testing or pre-implementation time. Ensuring a dedicated supervisor role and subject matter expert on hand, in this instance, was pivotal to a successful project, implementation and sustainability plan. Within 12 months of launch, the team had supported a total of 25 pleuroscopy procedures successfully with no safety threats identified or reported. This was a wonderful safety trend towards success for our redesign project. A formal assessment of wait times has not been performed although it is anticipated that wait times will be reduced, or at the least unchanged, given ongoing challenges with OR availability.
Our project and approach presented limitations. With a strong focus on the new and expanded scope for the RRTs, the initial skills training focused solely on their role. In hindsight, including other professionals earlier (i.e. Day 1 of workshop) such as the RNs and clerks in the simulations may have been advantageous to better understand the task interdependencies and interplay between all the roles involved in the cases. This came later, although it may have expedited and improved the systems-based learning to alter our initial approach. From a larger project perspective, it would have been helpful to collect more validated data on wait times, cost and safety data to have clear measures for change post-implementation in the new location.
System redesign projects in health care, such as the outpatient pleuroscopy project described in our article, should take special consideration to the proactive testing and training of teams embedding the use of simulation-based methods to ensure a safe, effective and efficient implementation. The potential impact on all system elements should be considered when making changes to one or many elements such as the environment, roles and responsibilities, tasks, staffing, organization and process. Whenever possible, simulation can and should be considered for a large role in quality improvement health system redesign projects.
The authors would like to thank all participating team members for supporting this work and the provincial simulation programme.
None declared.
There has been no funding for this work.
None declared.
None declared.
MD is faculty for Healthcare Systems Simulation International, which provides consulting and courses on systems-focused simulation and debriefing for quality and safety staff, simulation enthusiasts and others in health care. There are no competing interests.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.